A Fixation Protocol Compatible With R&D Systems Luminex Assays Enabling Analysis With Infectious Samples
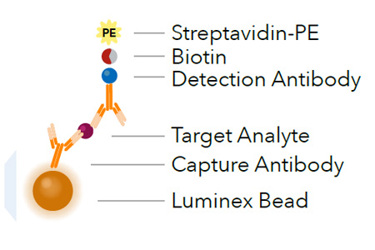
Multiplex protein analysis is a powerful research tool for immune monitoring and biomarker research. The bead-based multiplexing approach allows for analysis of multiple proteins with one test. This multiplex assay requires significantly less sample and time to set up than multiple traditional ELISAs, thus allowing for the measurement of the same number of analytes more quickly. The study of biological select agents such as bacteria, viruses, or toxins, typically requires Biosafety Level 3 (BSL3) or Biosafety Level 4 (BSL4) laboratories for containment. Research with these agents may also require expensive scientific instrumentation duplication. Because the use of shared instrumentation outside of BSL-3 or BSL-4 containment is a more cost effective option for researchers working with select agents, a process for the inactivation of infectious material is necessary for the safe transfer of samples and reagents to lower biosafety levels. A common way to inactivate infectious material is to use formaldehyde fixation. Confirmation of microorganism inactivation is accomplished using viability testing with broth or growth media for bacteria and plaque assays for viruses.1,2 Unfortunately, chemical fixation using formaldehyde has been shown to alter cytokines and chemokines in such a way that an immunoassay would not properly quantify biological samples.3 To address this issue, we decided to test the hypothesis that R&D Systems Luminex Assays are compatible with standard fixation protocols.
Get unlimited access to:
Enter your credentials below to log in. Not yet a member of Cell & Gene? Subscribe today.
