Patient Diversity In Clinical Studies: The Key To Advancing Precision Medicine
By Emily K. Pauli, Pharm.D.; Jeff Chen, MD/MBA; and Pelin Thorogood, M.Eng/MBA; Radicle Science

Precision medicine is about “delivering the right treatments, at the right time, every time to the right person,” as former President Obama aptly defined it. Large-scale longitudinal studies are necessary for precision medicine, but the constraints inherent to traditional clinical trials limit the understanding of key variables like gender, preexisting conditions, behavior, socioeconomics, race, and ethnicity.
Until we have better inclusion of participants with diverse demographics and behaviors – and can analyze that data in conjunction with prognostic data – we won’t know the effectiveness of a product for these large, underrepresented populations. Thankfully, crowdsourced virtual trials with intentional heterogeneity of participants are elegant solutions for getting us closer to precision medicine.
Heterogeneity Is Critical For Precision Care
Drug metabolism can differ across heterogeneous populations due to prognostic factors like age and gender, as well as allelic frequencies that tend to cluster by race/ethnicity. However, research often overlooks factors such as behavior and socioeconomic status. In a commentary in Nature, McCarthy and Birney argue that “clinical medicine must learn to develop more-holistic measures of individual risk, both genetic and non-genetic, and to combine these with clinical data over time to deliver better care.”
For medicine to become more precise, we must take into account all of the holistic data. As one example, numerous studies show genetics play a role in higher incidences of kidney disease among African Americans. This is why medicine uses more sensitive thresholds for screening and diagnosis of chronic kidney disease in African Americans, as “CKD progresses to end-stage renal disease far more rapidly in minority populations, with rates nearly four times higher in Black Americans than in white Americans.” However, there may be more at play here than alleles. “Socioeconomic factors and chronic stress, including the stress of racism, may increase the risk of having conditions that cause kidney disease — especially diabetes and high blood pressure.”
A holistic approach would give us invaluable insights that translate into precision care, and that’s precisely the approach the U.S. government is taking. The NIH is ambitiously combining both prognostic and demographic factors in a large-scale, intentionally heterogeneous, Big Data-driven study. The All of Us Research Program’s goal is to gather data from 1 million participants that reflect America’s diversity, especially from groups in biomedical research that have been underrepresented historically. The NIH believes: “Diversity in a research program is important for several reasons. First, where we live, how we live, and our background can all affect our health. Second, many groups of people have been left out of research in the past. This means researchers know less about their health.”
To advance medicine for all, we must advance research for all. As we increase diversity in our study populations, we expect to see differences in everything from health outcomes and adverse effects to consumption behavior. We also expect to see what’s unexpected, which is precisely why we need intentional heterogeneity in large-scale studies.
Barriers To Heterogeneity In Traditional Studies
Of course, scaling study size and achieving heterogeneity are easier said than done. Most traditional studies are limited to participants living within driving range of test clinics. The required time commitment and effort interfacing with the healthcare systems also dissuade tremendous numbers of potential participants. Unfortunately, the results of these studies are often from a homogenous, hyper-localized group of individuals who are comfortable with healthcare interactions and have the time to participate.
A study published in Clinical Investigation analyzed all of the trial sites registered in the U.S. FDA Bioresearch Monitoring Information System. It “revealed that the distribution of clinical trial sites per person was highly clustered around urban centers across the continental USA.”
Research shows that people want to participate in clinical trials, but they must be able to access them. A poll by Morning Consult found, “The American public is on board,” but with “roughly 4 in 5 adults saying it’s important that trials are easy for participants to get to and that they’re diverse.”
A few traditional solutions to this problem are to bring the study to the populace by establishing satellite clinics to test subjects close to their neighborhoods and/or hiring staff to administer in-home tests. These solutions work, but they are cumbersome and can cause costs to skyrocket.
The Morning Consult study elucidates, “Further, adults were more likely to say they would be interested in participating in a clinical trial if site visits were virtual or took place at retail clinics than if they were further than 30 minutes away or only took place at a hospital.”
With adults more willing to participate in virtual studies, these clearly become a forerunner in study design, especially when we consider their cost-effectiveness, scalability, timeliness, and potential for diversity.
Breaking Down Barriers With Virtual Studies
The authors of the study published in Clinical Investigation concluded that “expanding clinical research to rural areas may improve the heterogeneity of the sample population and generalizability of the research findings. Moreover, as research increasingly focuses on the effects of biologic and genetic variants on health outcomes and treatment efficacy, reaching a diversity of populations may be essential.”
They further noted “that geographic proximity is just one barrier to gaining access to clinical trials. Even when clinical trial sites exist nearby, gaining access to them can be difficult. For example, despite increased outreach efforts, racial and ethnic minority populations in urban areas remain underrepresented in clinical trials research.”
It is highly likely people in micropolitan or rural areas have limited access to clinics, and travel is a barrier. However, whether a prospective participant is a single, white farmer in rural Mississippi or a single Latin-X parent in New York City working full time for minimum wage, both share the same lack of access to healthcare and clinical trials. Those who are employed lose income when they are not working, and traditional trial sites rarely see participants after hours. Whether from loss of income, lack of time (and childcare), or limited geographic access, these are real barriers.
Achieving study heterogeneity representative of the American population requires meeting participants where they live, 100% of the time, whether they live in rural areas or work two jobs in the city. Our goal is to minimize the time commitment and eliminate forced interactions with healthcare systems. The only way to achieve this mission is to do so virtually, in an automated, low-touch fashion. Virtual studies remove geographic, socioeconomic, and even psychological barriers.
What We’re Doing To Address The Intentionality Of Diversity In Recruiting
In our nearly 3,000-participant Radicle ACES study – history’s largest real-world evidence randomized trial on CBD effectiveness – we filled our recruitment goal in 72 hours…however, we inadvertently filled it with middle-aged women representing a majority of our study population! For our next study, we wanted a more balanced representation of men and women. We achieved equal gender distribution for our pain study by oversampling for males initially through channels that predominantly reached men before opening recruitment through our other channels. That approach resulted in gender equality, with 51% women and 49% men in 1,800 participants enrolled in two weeks.
Our studies have diversity of ethnicity and race, and future ones will further focus on achieving diversity fully representative of the American population.
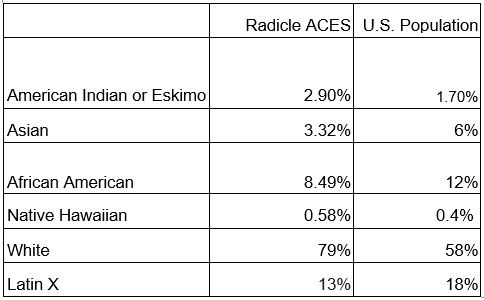
Virtual studies allow us to continually refine our recruitment strategy, targeting various ZIP codes and population groups until our intentional heterogeneity goals are reached.
With that study as well as our Radicle Real-World Sleep trial, there were 5,000 combined participants. Our studies were 100% remote and required no mandatory interactions with clinicians. Of these participants, 20% were located outside of metropolitan areas. This figure was not based solely on population density calculations but also incorporated traffic flows and commuting patterns to and from each ZIP code; this gave us a more accurate picture of these 20% of participants who would otherwise have very limited access to in-person trials.
We are proud that our virtual studies reflected the demographics seen in the most recent U.S. Census: nearly 20% of Americans live outside of metropolitan areas.
The participant feedback was compelling. One of the Radicle ACES study participants, who lives in a rural county, commented in a post-study survey: “It was exciting to be part of a study, which is something I've never done before and I would love to be considered again.”
Another participant, who lives in a micropolitan area, commented, “This was a pleasant experience. Very low pressure. Easy to maintain. I appreciate not having to deal with random phone calls. Especially during business hours when I can't answer the phone.”
We have great hope virtual studies will continue to open the door for people who otherwise could not be study participants. Averaging across our studies to date, 58.8% of our participants were employed. 16% were homemakers, 7.8% were unemployed, 5.3% were retired, and the remainder were students, unable to work, or declined to state.
The timing is perfect for crowdsourced virtual trials with intentional heterogeneity of participants. We have the technology, and with the adaptations COVID-19 thrust upon us, the populace is now highly acclimated to virtual technology.
Diversity Is In The Details: Considerations To Keep In Mind
Large-scale virtual studies share some of the same challenges as their more traditional counterparts. With all studies, even minute shifts in methodology have ramifications, especially in alienating entire socioeconomic or ethnic populations. Virtual studies must take these factors into account, as well as address their own unique challenges to inclusive methodology.
For instance, do we assume our participants have access to reliable internet? What happens if we require a smartphone vs. tablet or computer? What level of technological savvy are we assuming if we require participants to scan a QR code? Are there differences in sending links to surveys via text vs. email? What if we require a physical address vs. allow for a P.O. box? How are the results impacted if materials are available only in English?
We realize each assumption we make yields different results in the populations we target. This is where the intention comes into intentional heterogeneity. In upcoming studies, we will offer multiple ways for participants to engage, as we know that intentional heterogeneity cannot be a one-size-fits-all approach.
The Leap From Large-Scale Virtual Trials To Precision Medicine Is Standardization & Aggregation
We know highly diverse data holds the key to precision medicine, but the challenge is aggregating these large data sets across studies and accounting for all of the variables across outcomes. Standardization arises as a solution. As published in Frontiers in Medicine, authors spanning the Yale School of Medicine to Duke-NUS Medical School elaborate that for Big Data to become precision medicine, we must meet the challenge of “standardization of data content, format, and clinical definitions.”
This is precisely why Radicle Science conducts all our studies using common protocols and assessments, such as methodology from large-scale data initiatives like The Human Genome Project. In each study, we naturally collect standardized data across thousands of intentionally diverse participants. As we anonymize and aggregate these data sets, we get to explore hidden correlations among the rich dimensionality of our data across genetic, geographic, socioeconomic, environmental, behavioral, or cultural variables. The resulting predictive insights are as valuable as the depth and breadth of the data we collect, making us double down on our premise that large, intentionally heterogeneous studies can indeed power precision medicine.
Researchers from the Icahn School of Medicine at Mount Sinai and University of Texas Southwestern Medical Center elaborate that, “In the near future, much larger volumes and complex data sets for precision medicine will be generated, e.g., individual and longitudinal multi-omic, and direct-to-consumer data sets.”
That near future is now.
About the Authors:
 Emily K. Pauli, Pharm.D. is the CRO of Radicle Science. Throughout her 25-year career in research, she has worked tirelessly to streamline the research process, disrupt the status quo, and lessen the burden on trial participants. She is the 2022 recipient of the Health 2.0 Outstanding Leadership Award. Prior to joining Radicle Science as a founding member, she was director of research for Clearview Cancer Institute, where her leadership made it one of the few community cancer centers with a complete clinical research program for drug and diagnostic development. She received both her BS and Pharm. D. from Auburn University.
Emily K. Pauli, Pharm.D. is the CRO of Radicle Science. Throughout her 25-year career in research, she has worked tirelessly to streamline the research process, disrupt the status quo, and lessen the burden on trial participants. She is the 2022 recipient of the Health 2.0 Outstanding Leadership Award. Prior to joining Radicle Science as a founding member, she was director of research for Clearview Cancer Institute, where her leadership made it one of the few community cancer centers with a complete clinical research program for drug and diagnostic development. She received both her BS and Pharm. D. from Auburn University.
 Jeff Chen, MD/MBA, is the CEO and cofounder of Radicle Science. His vision to conduct research 10x cheaper and faster than traditional clinical trials is delivering one of history's first predictive and evidence-based health-decision data sets. He is also the founder and former executive director of the UCLA Cannabis Research Initiative, one of the first in the world. Under his leadership, the initiative grew to encompass more than 40 faculty conducting research, education, and policy projects. He is an industry mentor for the U.S. National Institutes of Health Innovation CORPs, a David Geffen Fellow, and advisor to Healthline. He earned his MD and MBA from UCLA, following a BSat from Cornell.
Jeff Chen, MD/MBA, is the CEO and cofounder of Radicle Science. His vision to conduct research 10x cheaper and faster than traditional clinical trials is delivering one of history's first predictive and evidence-based health-decision data sets. He is also the founder and former executive director of the UCLA Cannabis Research Initiative, one of the first in the world. Under his leadership, the initiative grew to encompass more than 40 faculty conducting research, education, and policy projects. He is an industry mentor for the U.S. National Institutes of Health Innovation CORPs, a David Geffen Fellow, and advisor to Healthline. He earned his MD and MBA from UCLA, following a BSat from Cornell.
 Pelin Thorogood, M.Eng/MBA, is cofounder and executive chair of Radicle Science. Thorogood is a tech executive turned impact entrepreneur. Previously, she was the CEO of Anametrix (acquired in 2015). She was a leader in creating the digital marketing space as CMO of WebSideStory (acquired by Adobe/Omniture in 2008). Thorogood is the trustee and treasurer of the UC San Diego Foundation, executive board member of the UC San Diego Basement start-up incubator, co-chair of the UC San Diego Innovation and Entrepreneurship Council, industry scholar for the Cornell Institute of Healthy Futures, and former executive-in-residence for the Johnson Graduate School of Management. She holds a BS M.Eng, and MBA degrees, all from Cornell.
Pelin Thorogood, M.Eng/MBA, is cofounder and executive chair of Radicle Science. Thorogood is a tech executive turned impact entrepreneur. Previously, she was the CEO of Anametrix (acquired in 2015). She was a leader in creating the digital marketing space as CMO of WebSideStory (acquired by Adobe/Omniture in 2008). Thorogood is the trustee and treasurer of the UC San Diego Foundation, executive board member of the UC San Diego Basement start-up incubator, co-chair of the UC San Diego Innovation and Entrepreneurship Council, industry scholar for the Cornell Institute of Healthy Futures, and former executive-in-residence for the Johnson Graduate School of Management. She holds a BS M.Eng, and MBA degrees, all from Cornell.
