Key Considerations For Labeling Your Cell Or Gene Therapy
By Abdul Ally and Amy Hendricks, Thermo Fisher Scientific
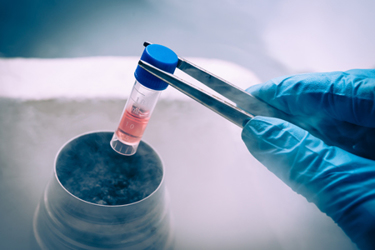
With the continued expansion of the pharmaceutical industry’s global footprint and the growing complexity of today’s drug products, ensuring their safe delivery to patients is becoming more challenging than ever before. Not only must a sponsor secure a supply chain that can support their commercial goals, but they must also safeguard their products using viable packaging and labeling strategies. Without them, they run the risk of their drug being delivered outside of its specifications and/or without the information necessary for it to be properly identified and used during clinical trials.
This can be particularly difficult for those products shipped within the cryogenic temperature range of -150° to -196° C, such as cell and gene therapies. Time out of temperature can have a major impact on the efficacy of the therapy and, ultimately, its clinical outcomes. As sponsors focus on maintaining the temperature range required for these therapies, they must also keep in mind the challenges these temperatures can present when properly labeling personalized therapies for clinical trial distribution.
Get unlimited access to:
Enter your credentials below to log in. Not yet a member of Cell & Gene? Subscribe today.
